In-stent restenosis in coronary arteries – computational and data-driven investigations towards translational modeling
PIs: Marek Behr, Kevin Linka, Felix Vogt
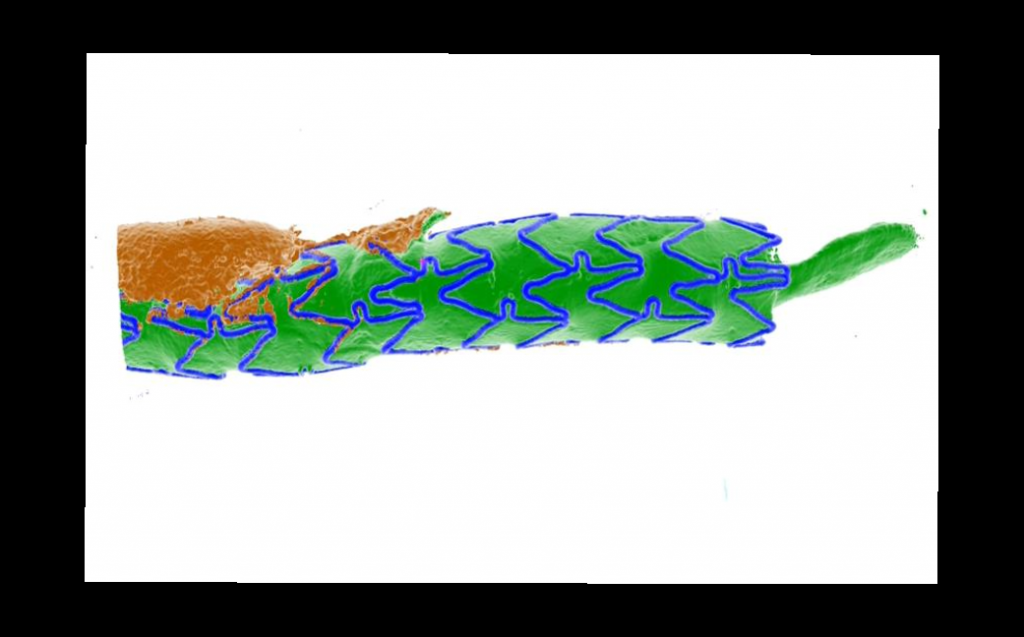
3-D reconstruction of a stented coronary artery from post-mortem µCT imaging (CARD): blue – stent; green – lumen; brown – calcification.
Aim:
The project develops computational tools for coronary stenting with drug elution to prevent in-stent restenosis. It combines medical data, cellular modeling, and blood flow dynamics to create a rapid, patient-specific simulation tool using techniques like model order reduction and neural networks.
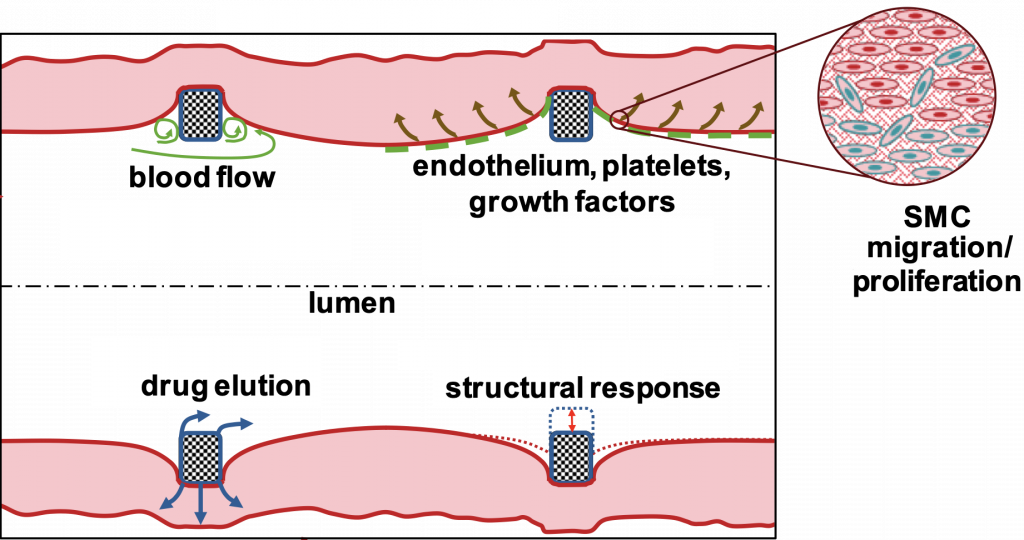
Graphical abstract of coronary stent project project
Description:
The project aims to develop computational and data-driven techniques for coronary stenting with drug elution to prevent the development of in-stent restenosis (ISR), a disease caused by pathological tissue growth. The aim is to create an in-silico simulation tool to help cardiologists make rapid, patient-specific treatment decisions. The project involves a multidisciplinary team: Prof. Vogt provides medical data and knowledge on ISR, Prof. Linka models cellular processes, and Prof. Behr focuses on the dynamics of blood flow. Using techniques such as model order reduction and neural networks, they aim to develop a meta-model that predicts ISR outcomes based on patient-specific data.
Involved Institutions:
Chair for Computational Analysis of Technical Systems, RWTH Aachen University
Department of Internal Medicine I – Cardiology, Angiology and Intensive Care Medicine, Uniklinik RWTH Aachen
Institute for Continuum and Material Mechanics, Hamburg University of Technology
Institute of Applied Mechanics, RWTH Aachen University
Links:
Applicants:
Prof. Marek Behr, Ph.D.
Dr.-Ing. Kevin Linka
Prof. Dr. med. Felix Vogt
Anna M. Ranno
Mahmoud Sesa
Publications
2025
Sesa, Mahmoud; Holthusen, H.; Böhm, C.; Jockenhövel, S.; Reese, Stefanie; Linka, Kevin
In: Biomechanics and Modeling in Mechanobiology, Bd. 24, S. 1687–1711, 2025.
@article{Sesa2025,
title = {A comprehensive framework for computational modeling of growth and remodeling in tissue-engineered soft collagenous materials},
author = {Mahmoud Sesa and H. Holthusen and C. Böhm and S. Jockenhövel and Stefanie Reese and Kevin Linka},
editor = {Springer nature },
url = {https://link.springer.com/article/10.1007/s10237-025-01988-x},
doi = {https://doi.org/10.1007/s10237-025-01988-x},
year = {2025},
date = {2025-07-23},
urldate = {2025-07-23},
journal = { Biomechanics and Modeling in Mechanobiology},
volume = {24},
pages = {1687–1711},
keywords = {},
pubstate = {published},
tppubtype = {article}
}
2024
Ranno, A.; Manjunatha, K.; Glitz, A.; Schaaps, N.; Reese, S.; Vogt, F.; Behr, M.
In-silico Analysis of Hemodynamic Indicators in Idealized Stented Coronary Arteries for Varying Stent Indentation Unveröffentlicht
2024.
@unpublished{Ranno2024,
title = {In-silico Analysis of Hemodynamic Indicators in Idealized Stented Coronary Arteries for Varying Stent Indentation},
author = {A. Ranno and K. Manjunatha and A. Glitz and N. Schaaps and S. Reese and F. Vogt and M. Behr},
editor = {Cornell University},
url = {https://arxiv.org/abs/2401.08701},
doi = {10.48550/arXiv.2401.08701},
year = {2024},
date = {2024-01-14},
urldate = {2024-01-14},
abstract = {In this work, we investigate the effects of stent indentation on hemodynamic indicators in stented coronary arteries. Our aim is to assess in-silico risk factors for in-stent restenosis (ISR) and thrombosis after stent implantation. The proposed model is applied to an idealized artery with Xience V stent for four indentation percentages and three mesh refinements. We analyze the patterns of hemodynamic indicators arising from different stent indentations and propose an empirical frequency analysis of time-averaged WSS (TAWSS), oscillatory shear index (OSI), and relative residence time (RRT). We observe that higher indentations display higher frequency of critically low TAWSS and non-physiological OSI and RRT. Furthermore, an appropriate mesh refinement is needed for accurate representation of hemodynamics in the stent vicinity. The results provide physics-based evidence for the correlation between high indentation and ISR.},
keywords = {},
pubstate = {published},
tppubtype = {unpublished}
}
Shi, Jianye; Manjunatha, Kiran; Behr, Marek; Vogt, Felix; Reese, Stefanie
A physics-informed deep learning framework for modeling of coronary in-stent restenosis Artikel
In: Biomechanics and Modeling in Mechanobiology, Bd. 23, Nr. 2, S. 615–629, 2024.
@article{shi2024a,
title = {A physics-informed deep learning framework for modeling of coronary in-stent restenosis},
author = {Jianye Shi and Kiran Manjunatha and Marek Behr and Felix Vogt and Stefanie Reese},
year = {2024},
date = {2024-01-01},
urldate = {2024-01-01},
journal = {Biomechanics and Modeling in Mechanobiology},
volume = {23},
number = {2},
pages = {615–629},
publisher = {Springer},
keywords = {},
pubstate = {published},
tppubtype = {article}
}
Shi, Jianye; Manjunatha, Kiran; Vogt, Felix; Reese, Stefanie
Data-driven reduced order surrogate modeling for coronary in-stent restenosis Artikel
In: Computer Methods and Programs in Biomedicine, S. 108466, 2024.
@article{shi2024data,
title = {Data-driven reduced order surrogate modeling for coronary in-stent restenosis},
author = {Jianye Shi and Kiran Manjunatha and Felix Vogt and Stefanie Reese},
year = {2024},
date = {2024-01-01},
urldate = {2024-01-01},
journal = {Computer Methods and Programs in Biomedicine},
pages = {108466},
publisher = {Elsevier},
keywords = {},
pubstate = {published},
tppubtype = {article}
}
2023
Manjunatha, K.; Schaaps, N.; Behr, M.; Vogt, F.; Reese, S.
Computational Modeling of In-Stent Restenosis: Pharmacokinetic and Pharmacodynamic Evaluation Artikel
In: Computers in Biology and Medicine, Ausg. 167, 2023.
@article{Manjunatha2023,
title = {Computational Modeling of In-Stent Restenosis: Pharmacokinetic and Pharmacodynamic Evaluation},
author = {K. Manjunatha and N. Schaaps and M. Behr and F. Vogt and S. Reese},
editor = {National Library Medicine},
url = {https://pubmed.ncbi.nlm.nih.gov/37972534/},
doi = {10.1016/j.compbiomed.2023.107686},
year = {2023},
date = {2023-12-01},
urldate = {2023-12-01},
journal = {Computers in Biology and Medicine},
issue = {167},
abstract = {Persistence of the pathology of in-stent restenosis even with the advent of drug-eluting stents warrants the development of highly resolved in silico models. These computational models assist in gaining insights into the transient biochemical and cellular mechanisms involved and thereby optimize the stent implantation parameters. Within this work, an already established fully-coupled Lagrangian finite element framework for modeling the restenotic growth is enhanced with the incorporation of endothelium-mediated effects and pharmacological influences of rapamycin-based drugs embedded in the polymeric layers of the current generation drug-eluting stents. The continuum mechanical description of growth is further justified in the context of thermodynamic consistency. Qualitative inferences are drawn from the model developed herein regarding the efficacy of the level of drug embedment within the struts as well as the release profiles adopted. The framework is then intended to serve as a tool for clinicians to tune the interventional procedures patient-specifically.},
keywords = {},
pubstate = {published},
tppubtype = {article}
}
Cornelissen, A.; Florescu, R. A.; Reese, S.; Behr, M.; Ranno, A.; Manjunatha, K.; Schaaps, N.; Böhm, C.; Liehn, E. A.; Zhao, L.; Nilcham, P.; Milzi, A.; Schröder, J.; Vogt, F. J.
In-Vivo Assessment of Vascular Injury for the Prediction of In-Stent Restenosis Artikel
In: International Journal of Cardiology, Ausg. 388, 2023.
@article{Cornelissen2023,
title = {In-Vivo Assessment of Vascular Injury for the Prediction of In-Stent Restenosis},
author = {A. Cornelissen and R. A. Florescu and S. Reese and M. Behr and A. Ranno and K. Manjunatha and N. Schaaps and C. Böhm and E. A. Liehn and L. Zhao and P. Nilcham and A. Milzi and J. Schröder and F. J. Vogt},
editor = {National Library Medicine},
url = {https://pubmed.ncbi.nlm.nih.gov/37423572/},
doi = {10.1016/j.ijcard.2023.131151},
year = {2023},
date = {2023-07-07},
urldate = {2023-07-07},
journal = {International Journal of Cardiology},
issue = {388},
abstract = {Background: Despite optimizations of coronary stenting technology, a residual risk of in-stent restenosis (ISR) remains. Vessel wall injury has important impact on the development of ISR. While injury can be assessed in histology, there is no injury score available to be used in clinical practice.
Methods: Seven rats underwent abdominal aorta stent implantation. At 4 weeks after implantation, animals were euthanized, and strut indentation, defined as the impression of the strut into the vessel wall, as well as neointimal growth were assessed. Established histological injury scores were assessed to confirm associations between indentation and vessel wall injury. In addition, stent strut indentation was assessed by optical coherence tomography (OCT) in an exemplary clinical case. Results: Stent strut indentation was associated with vessel wall injury in histology. Furthermore, indentation was positively correlated with neointimal thickness, both in the per-strut analysis (r = 0.5579) and in the per-section analysis (r = 0.8620; both p ≤ 0.001). In a clinical case, indentation quantification in OCT was feasible, enabling assessment of injury in vivo.
Conclusion: Assessing stent strut indentation enables periprocedural assessment of stent-induced damage in vivo and therefore allows for optimization of stent implantation. The assessment of stent strut indentation might become a valuable tool in clinical practice.
Keywords: In stent restenosis; Optical coherence tomography; Patient-individualized percutaneous coronary intervention; Prediction; Stent malapposition.},
keywords = {},
pubstate = {published},
tppubtype = {article}
}
Methods: Seven rats underwent abdominal aorta stent implantation. At 4 weeks after implantation, animals were euthanized, and strut indentation, defined as the impression of the strut into the vessel wall, as well as neointimal growth were assessed. Established histological injury scores were assessed to confirm associations between indentation and vessel wall injury. In addition, stent strut indentation was assessed by optical coherence tomography (OCT) in an exemplary clinical case. Results: Stent strut indentation was associated with vessel wall injury in histology. Furthermore, indentation was positively correlated with neointimal thickness, both in the per-strut analysis (r = 0.5579) and in the per-section analysis (r = 0.8620; both p ≤ 0.001). In a clinical case, indentation quantification in OCT was feasible, enabling assessment of injury in vivo.
Conclusion: Assessing stent strut indentation enables periprocedural assessment of stent-induced damage in vivo and therefore allows for optimization of stent implantation. The assessment of stent strut indentation might become a valuable tool in clinical practice.
Keywords: In stent restenosis; Optical coherence tomography; Patient-individualized percutaneous coronary intervention; Prediction; Stent malapposition.
Manjunatha, Kiran; Ranno, Anna; Shi, Jianye; Schaaps, Nicole; Nilcham, Pakhwan; Cornelissen, Anne; Vogt, Felix; Behr, Marek; Reese, Stefanie
In: GAMM-Mitteilungen, Bd. n/a, Nr. n/a, S. e202370008, 2023.
@article{Manjunatha2023a,
title = {A continuum chemo-mechano-biological model for in-stent restenosis with consideration of hemodynamic effects},
author = {Kiran Manjunatha and Anna Ranno and Jianye Shi and Nicole Schaaps and Pakhwan Nilcham and Anne Cornelissen and Felix Vogt and Marek Behr and Stefanie Reese},
doi = {https://doi.org/10.1002/gamm.202370008},
year = {2023},
date = {2023-00-00},
urldate = {2023-00-00},
journal = {GAMM-Mitteilungen},
volume = {n/a},
number = {n/a},
pages = {e202370008},
keywords = {},
pubstate = {published},
tppubtype = {article}
}
2022
Manjunatha, K.; Behr, M.; Vogt, F.; Reese, S.
In: Computers in Biology and Medicine, Bd. 150, 2022.
@article{Manjunatha2022b,
title = {A Multiphysics Modeling Approach for In-Stent Restenosis: Theoretical Aspects and Finite Element Implementation},
author = {K. Manjunatha and M. Behr and F. Vogt and S. Reese},
editor = {Elsevier},
url = {https://www.sciencedirect.com/science/article/pii/S0010482522008745},
doi = {10.1016/j.compbiomed.2022.106166},
year = {2022},
date = {2022-11-15},
urldate = {2022-11-15},
journal = {Computers in Biology and Medicine},
volume = {150},
abstract = {Development of in silico models that capture progression of diseases in soft biological tissues are intrinsic in the validation of the hypothesized cellular and molecular mechanisms involved in the respective pathologies. In addition, they also aid in patient-specific adaptation of interventional procedures. In this regard, a fully-coupled high-fidelity Lagrangian finite element framework is proposed within this work which replicates the pathology of in-stent restenosis observed post stent implantation in a coronary artery. Advection–reaction–diffusion equations are set up to track the concentrations of the platelet-derived growth factor, the transforming growth factor-, the extracellular matrix, and the density of the smooth muscle cells. A continuum mechanical description of volumetric growth involved in the restenotic process, coupled to the evolution of the previously defined vessel wall constituents, is presented. Further, the finite element implementation of the model is discussed, and the behavior of the computational model is investigated via suitable numerical examples. Qualitative validation of the computational model is presented by emulating a stented artery. Patient-specific data are intended to be integrated into the model to predict the risk of in-stent restenosis, and thereby assist in the tuning of stent implantation parameters to mitigate the risk.},
keywords = {},
pubstate = {published},
tppubtype = {article}
}
Manjunatha, K.; Behr, M.; Vogt, F.; Reese, S.
Finite Element Modeling of In-Stent Restenosis Buchabschnitt
In: Link, Springer (Hrsg.): S. 305–318, 2022.
@incollection{Manjunatha2022,
title = {Finite Element Modeling of In-Stent Restenosis},
author = {K. Manjunatha and M. Behr and F. Vogt and S. Reese},
editor = {Springer Link},
url = {https://link.springer.com/chapter/10.1007/978-3-030-87312-7_30},
doi = {10.1007/978-3-030-87312-7_30},
year = {2022},
date = {2022-03-13},
urldate = {2022-03-13},
pages = {305–318},
abstract = {From the perspective of coronary heart disease, the development of stents has come significantly far in reducing the associated mortality rate, drug-eluting stents being the epitome of innovative and effective solutions. Within this work, the intricate process of in-stent restenosis is modelled considering one of the significant growth factors and its effect on constituents of the arterial wall. A multiphysical modelling approach is adopted in this regard. Experimental investigations from the literature have been used to hypothesize the governing equations and the corresponding parameters. A staggered solution strategy is utilised to capture the transport phenomena as well as the growth and remodeling that follows stent implantation. The model herein developed serves as a tool to predict in-stent restenosis depending on the endothelial injury sustained and the protuberance of stents into the lumen of the arteries.},
keywords = {},
pubstate = {published},
tppubtype = {incollection}
}
2020
Haßler, S.; Ranno, A.; Behr, M.
In: CMAME, Ausg. 369, 2020.
@article{Haßler2020,
title = {Finite-Element Formulation for Advection–Reaction Equations with Change of Variable and Discontinuity Capturing},
author = {S. Haßler and A. Ranno and M. Behr},
editor = {Elsevier},
url = {https://www.sciencedirect.com/science/article/pii/S004578252030356X},
doi = {10.1016/j.cma.2020.113171},
year = {2020},
date = {2020-06-11},
urldate = {2020-06-11},
journal = {CMAME},
issue = {369},
abstract = {We propose a change of variable approach and discontinuity capturing methods to ensure physical constraints for advection–reaction equations discretized by the finite element method. This change of variable confines the concentration below an upper bound in a very natural way. For the non-negativity constraint, we propose to use a discontinuity capturing method defined on the reference element that is combined with an anisotropic crosswind-dissipation operator. This discontinuity capturing cannot completely eliminate negative values but effectively minimizes their occurrence. The proposed methods are applied to different biophysical models and show a good agreement with experimental results for the FDA benchmark blood pump for a physiological red blood cell pore formation model.},
keywords = {},
pubstate = {published},
tppubtype = {article}
}
